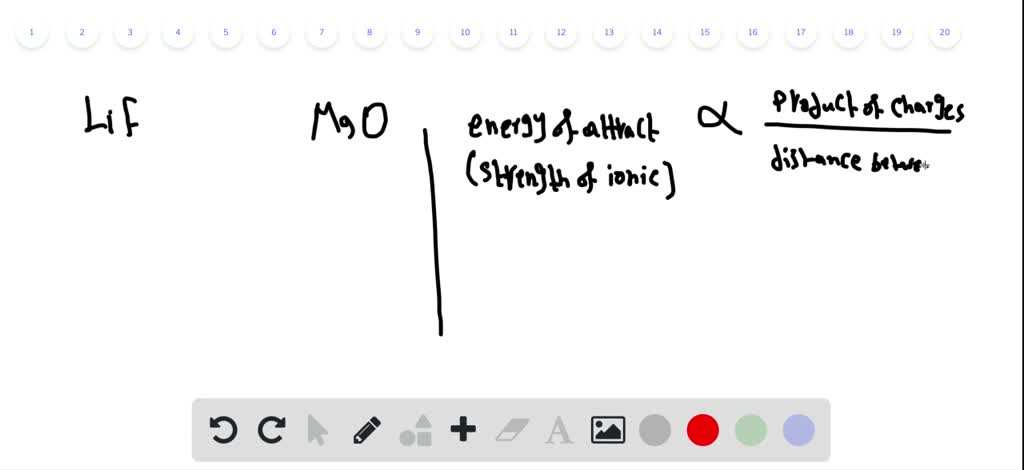
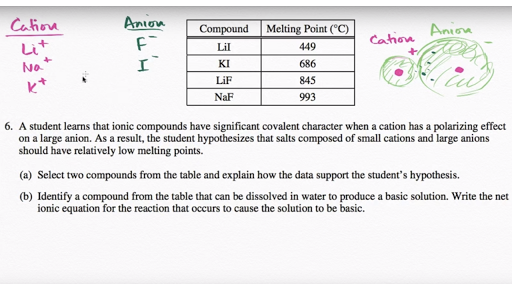
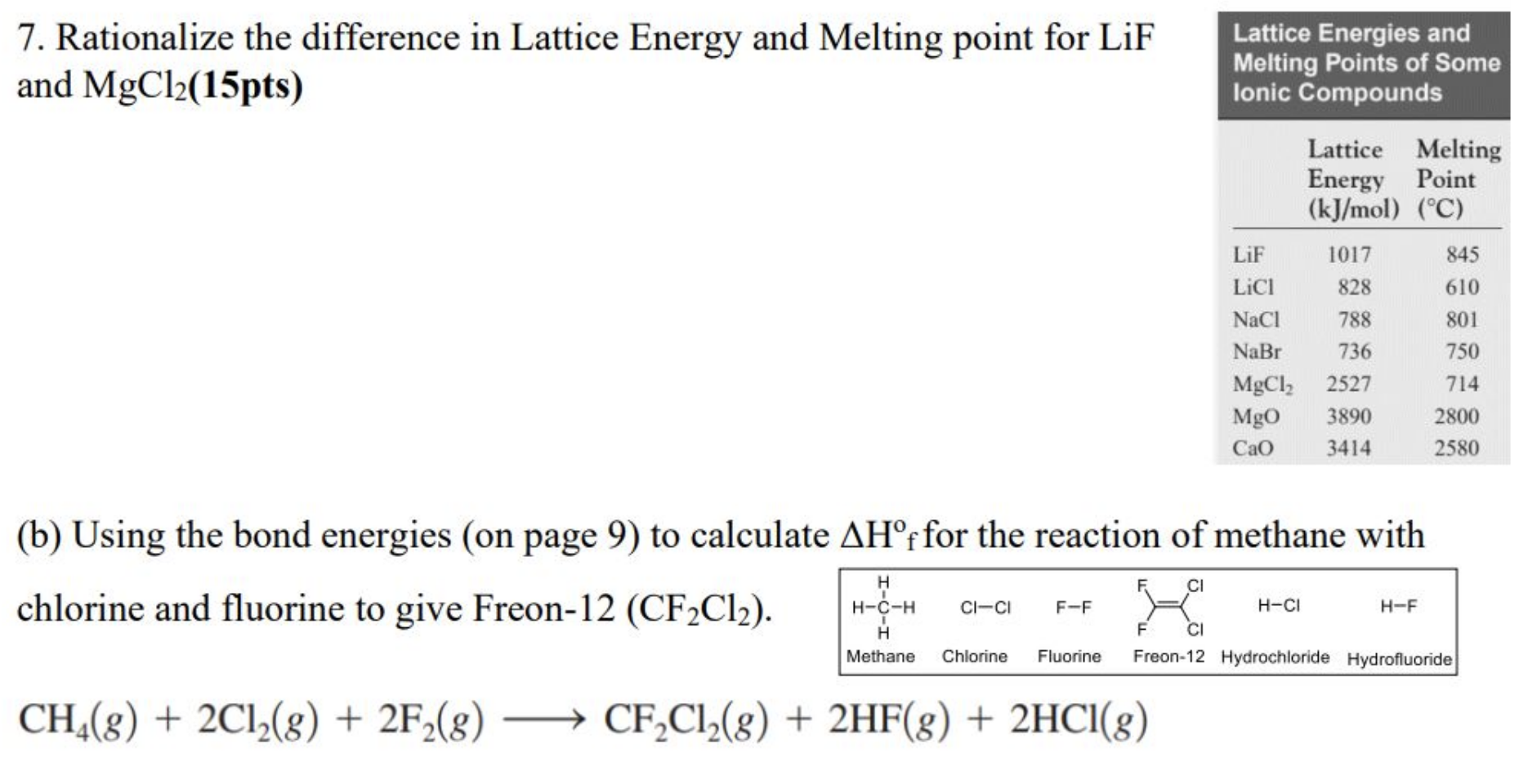
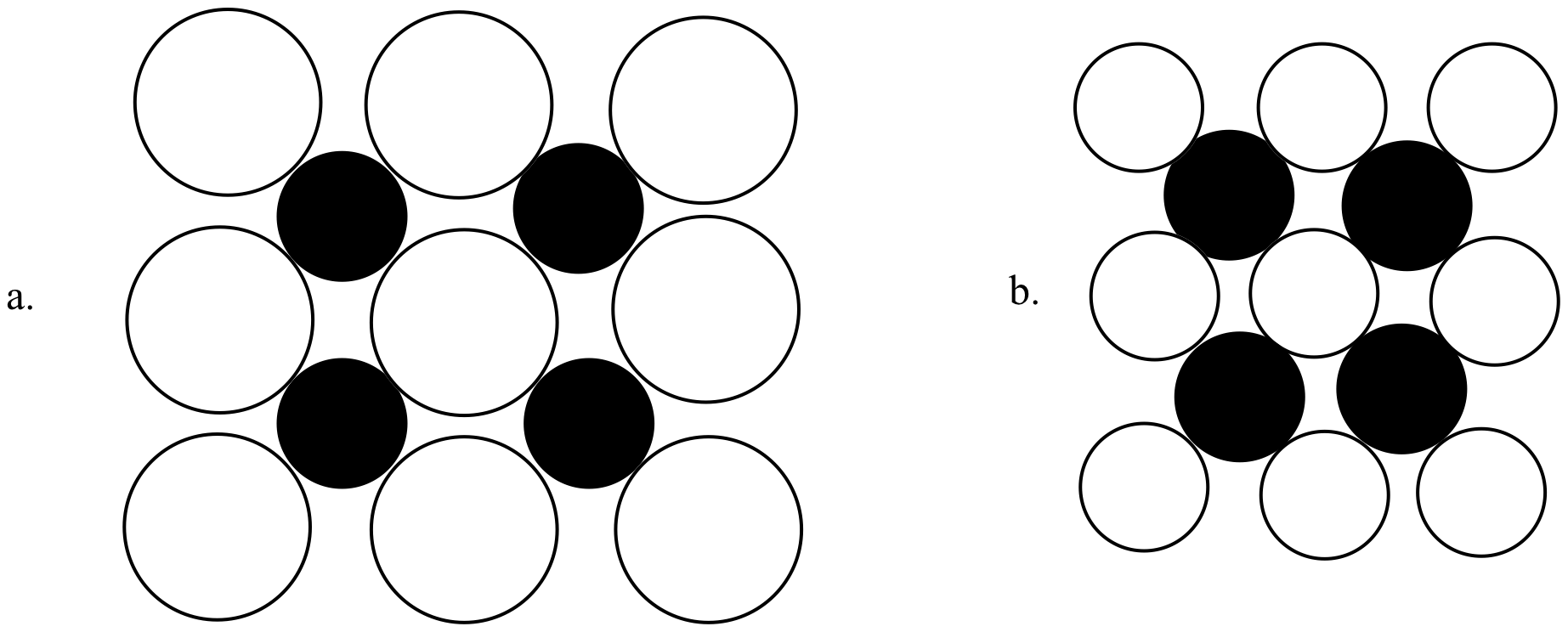
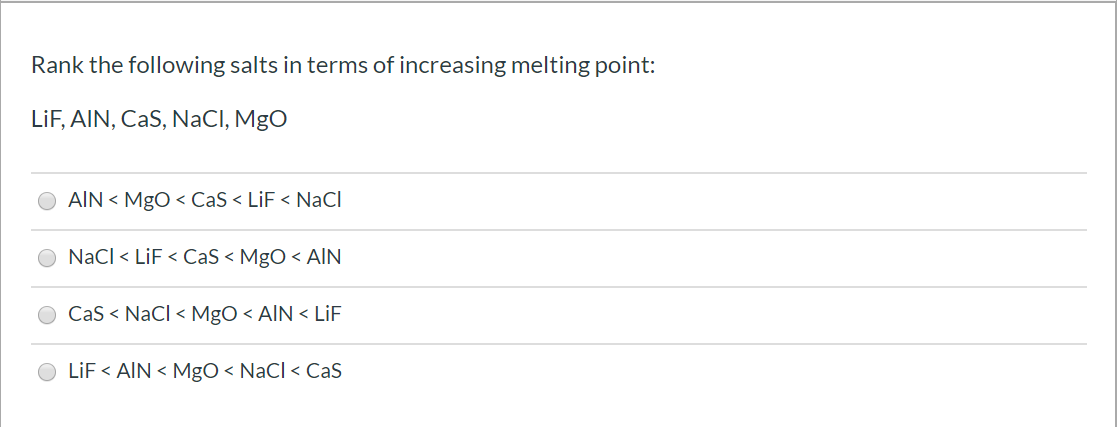
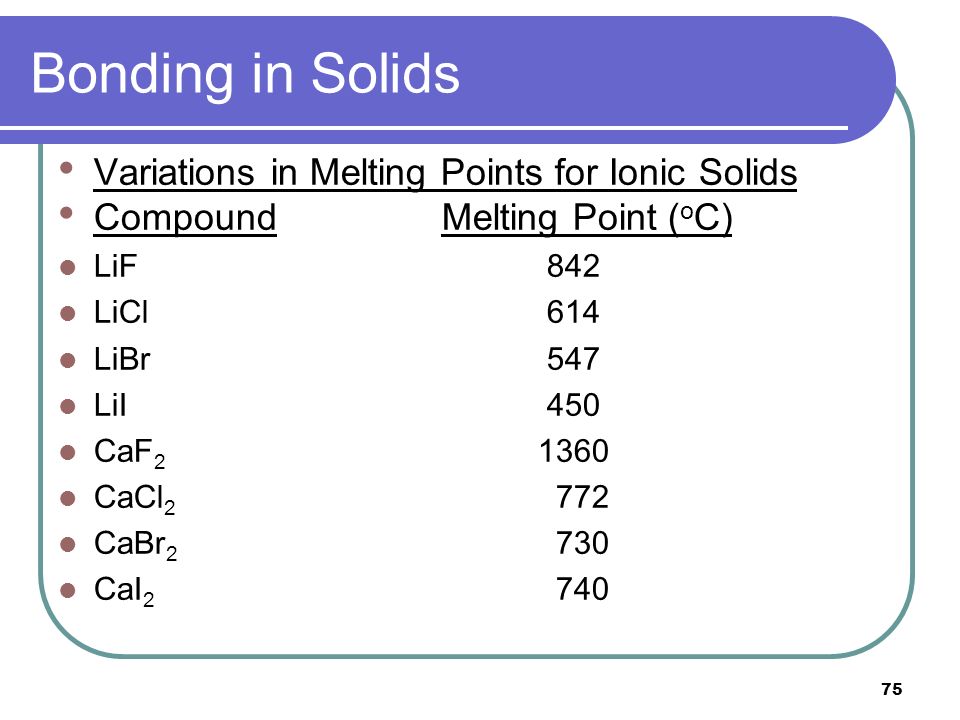
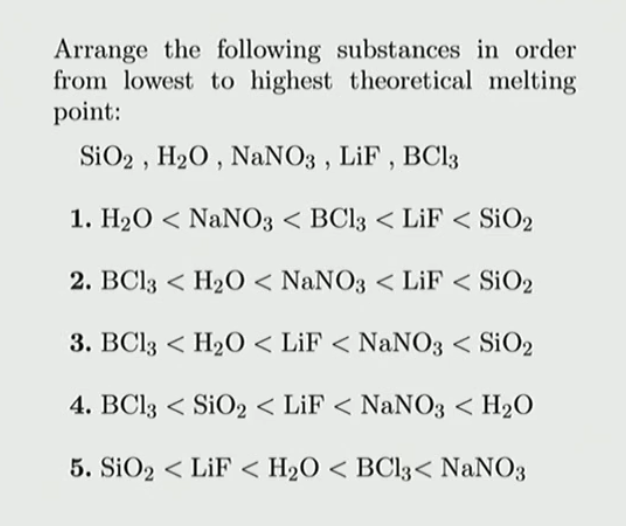
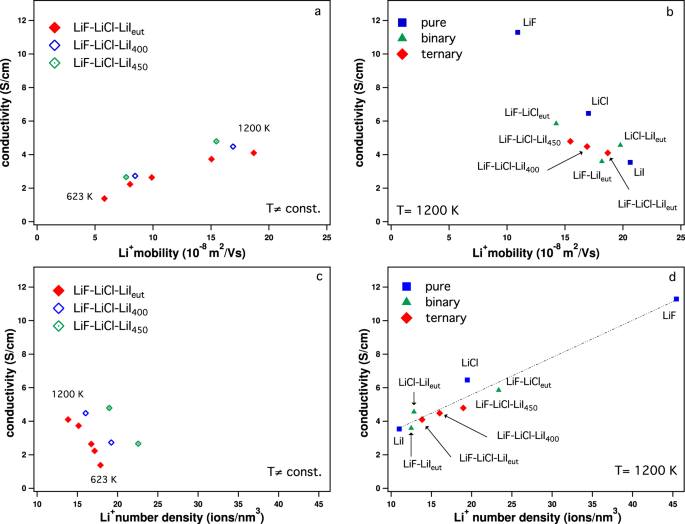
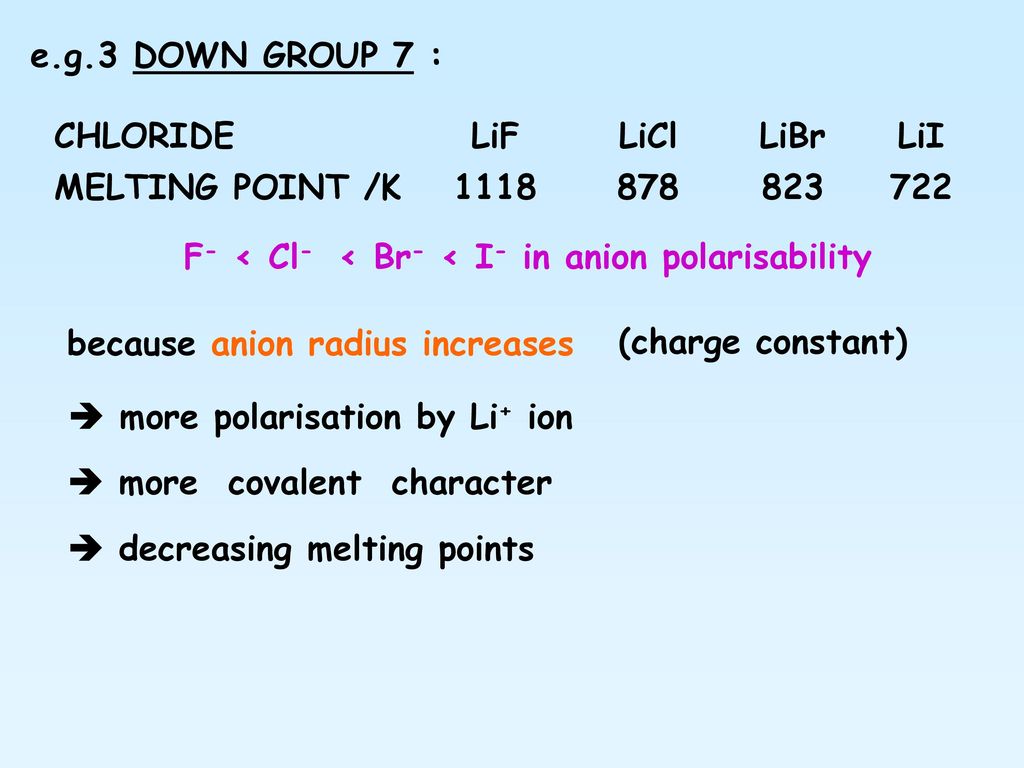
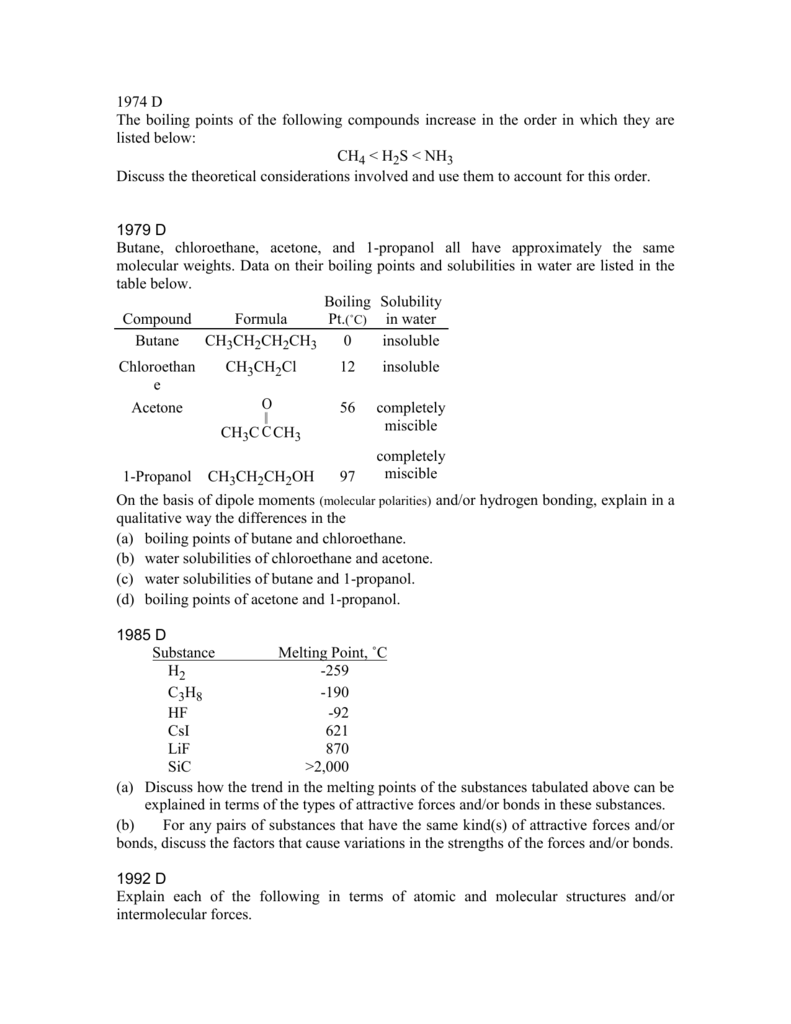
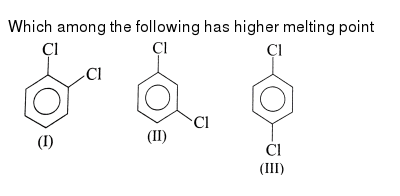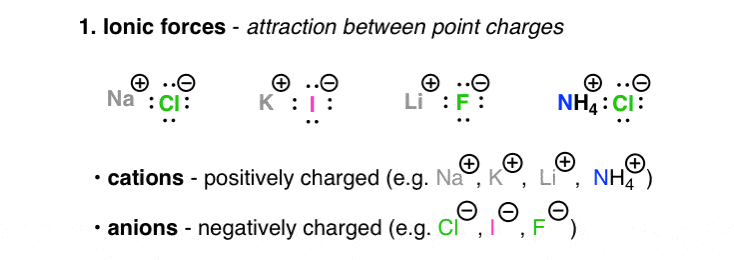lif melting point
2oh ch 3ch 2ch 2ch 3 ch 3ch 2och 3 29. Ch 3ch 2ch 2nh 2 licl n ch 3 3 ch 3ch 2ch 2ch 2ch 2nh 2 30. Boiling point the temperature at which a liquid turns into a gas.
Ethanol would have a higher boiling point than heptane e.

Lif melting point. Ethanol ch 3ch 2oh mw 46 has a boiling point of 78º. 2 lif cl 2 ch 3oh 61 26 70 32. 14 mw 86 has a boiling point of 68º. Industrial fires may reach higher temperatures but tests have shown that the molten cdte remains contained in the pv module.
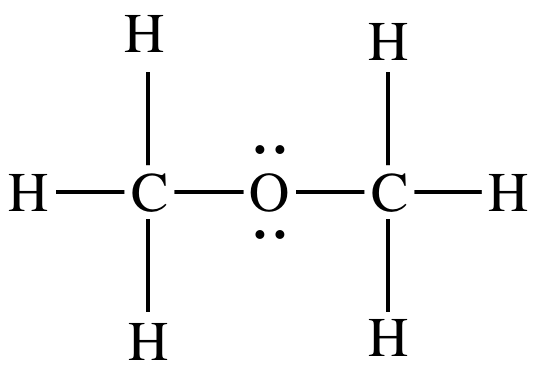
Ethanol ch 3 ch 2 oh is the product of the hydration reaction of ethene ch 2 ch 2 formed by the addition of h to one c and oh to the other c and so can be considered as the. Shop for the shark zu561 navigator lift away speed self cleaning brushroll lightweight upright vacuum with hepa filter red peony at the amazon home kitchen store. Elemental fluorine was first discovered in 1886 by isolating it from hydrofluoric acid fluorine exists as a diatomic molecule in its free state f 2 and is the most abundant halogen found in the earth s crust fluorine is the most electronegative element in the periodic table. It is also worth noting that one nicd battery contains 2500 times as much cadmium as a thin film cdte pv module and the.
H and oh to a molecular entity. The electrons in the outermost shell are the valence electrons the electrons on an atom that can be gained or lost in a chemical reaction. Mark each of the following statements as true or false. 845 c 1 553 f.
Acetone would have a higher boiling point than heptane. 25 939 2 g mol appearance white powder or transparent crystals hygroscopic. The melting point of cdte is 1050 degrees c so accidental domestic fires would not pose a risk. Melting point the temperature at which a solid turns into a liquid.
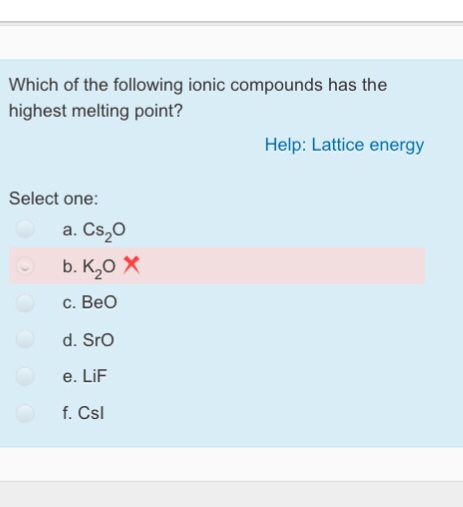
Fluorine fluorine has an atomic number of 9 and is denoted by the symbol f. Addition of water or of the elements of water i e. Rank the melting points for the following 1 being highest. The electrons on an atom that are not present in the previous rare gas ignoring filled d or f subshells.
See standard state and enthalpy of formation gibbs free energy of formation entropy and heat capacity for thermodynamic data for the same compounds. Since filled d or f subshells are seldom disturbed in a chemical reaction we can define valence electrons as follows. Ethanol has a higher boiling point because of greater london dispersion force c. The reason why ce naf would have the highest melting point is because if you look at the electronegativity values fluorine has the highest hence it have the greatest pull or attraction towards the electrons in the ionic bond resulting in a really strong sodium positive ion and a strong negative fluorine ion this thereby results in a high.
Chemical nature organic chemistry. Find products from shark with the lowest prices. Ethanol must have stronger intermolecular attraction based on its higher boiling point.