
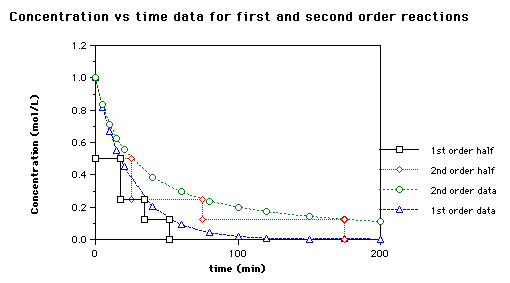
first order half life graph

half life for zero order reaction a g

period of 69 3sec at 0 10mol l

solved a certain first order reaction

first order reaction and its half life

if half life of a reaction is halved as
activation energy

rate constant k 5 5 10raised

the half life of a first order reaction
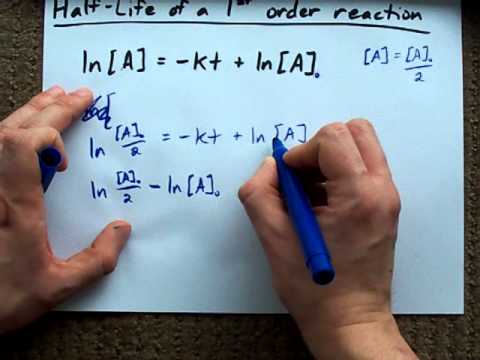
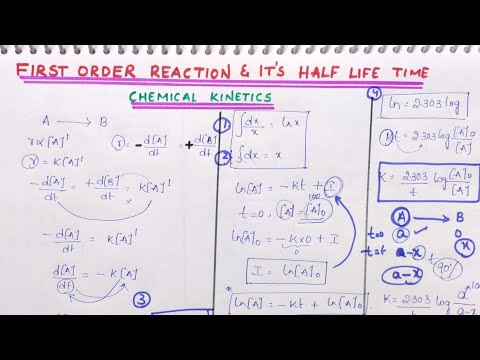
half life of a first order reaction derivation

the half life of a first order reaction

half life period of a reaction

the half life for a first order reaction is 50 sec when a o 0 84 mol l 1 the time needed for the concentration of a to decrease to one fourth of its original concentration is a 100 sec b 150

brainkart
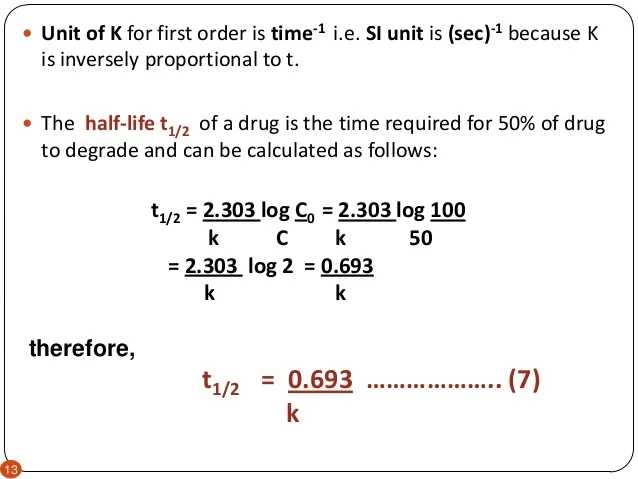
order reaction s j shah
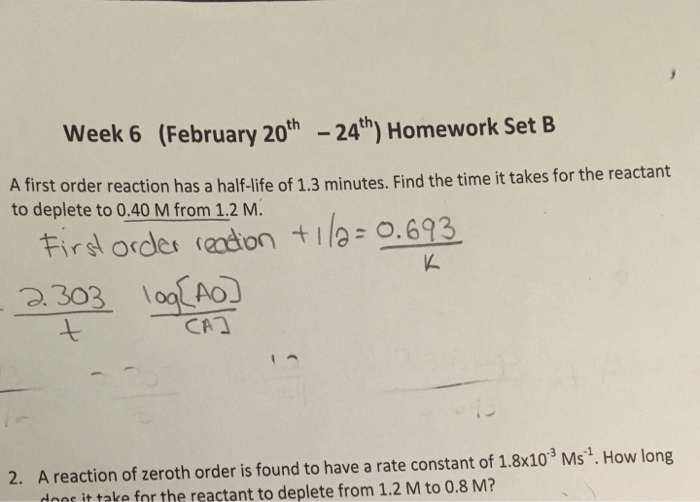
a first order reaction has a half life
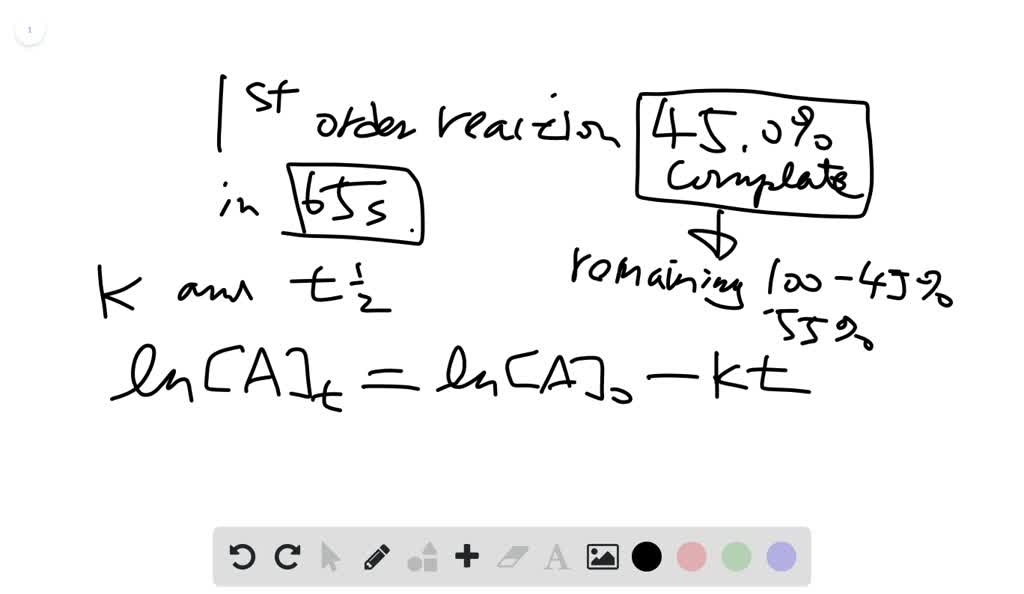
a certain first order reaction is 450 complete in 65 s what are the values of the rate constant and
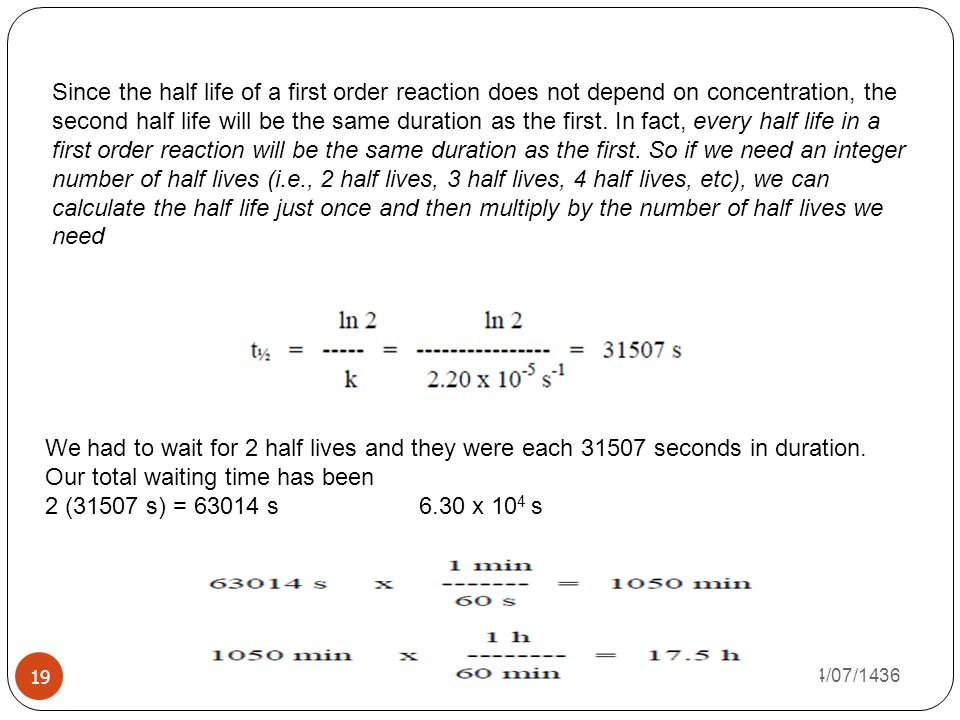
kinetics of drug stability phr 416

half life of a first order reaction
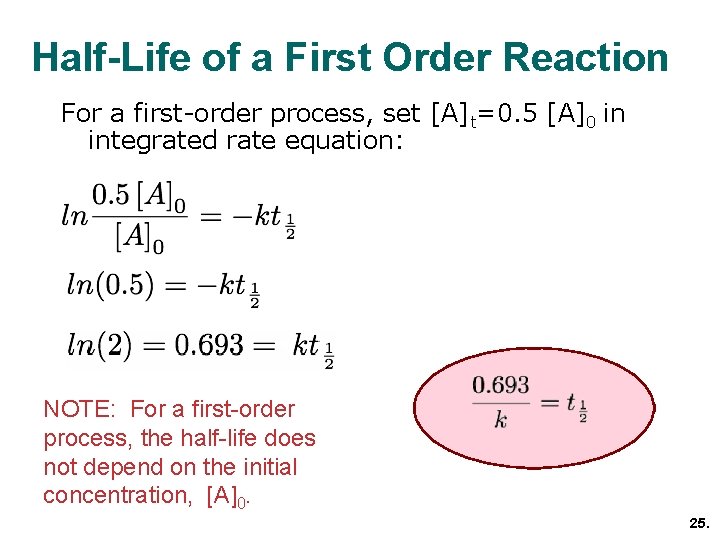
chemical kinetics chapter 14 chemistry

half life t 1 of the first order

pseudo first order reaction rate

chemical kinetics rates at which
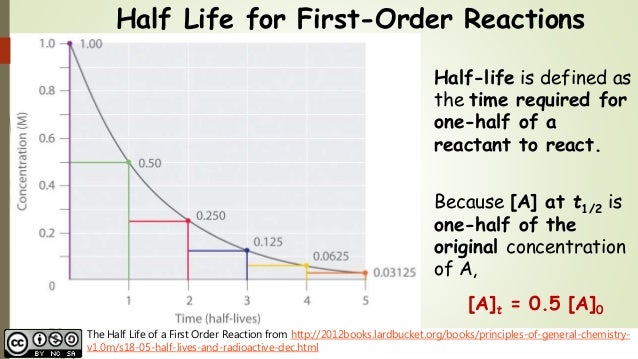
the first order integrated rate law

and first order reaction rate constants

order reaction is 10 minutes starting
topperlearning

first order reaction is 60 min
first order reaction derivation and it s half life time chemical kinetics chapter
You May Like